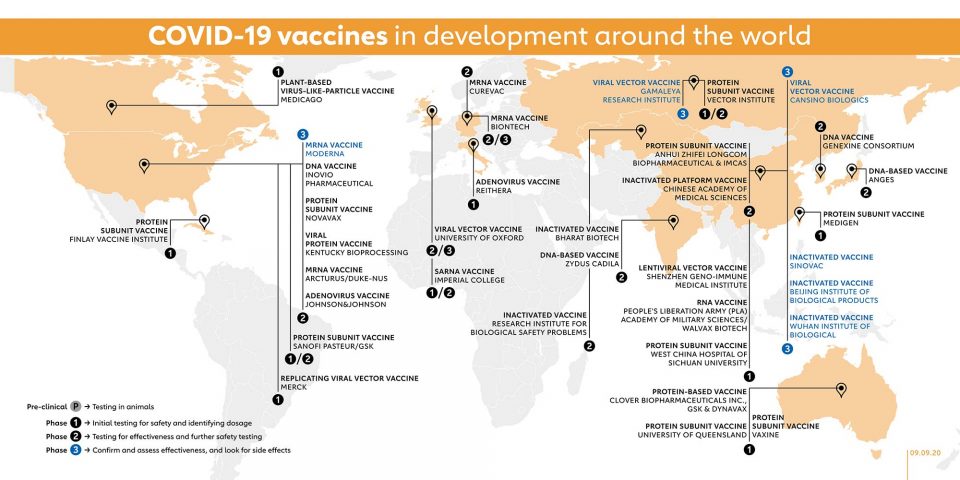
When candidate vaccines make it to human clinical trials, they first go through phase 1 trials primarily to test the vaccine’s safety, determine dosages and identify any potential side effects in a small number of people. Phase 2 trials further explore safety and start to investigate efficacy in larger groups. The final stage, phase 3 trials, which few vaccines ever make it to, involve thousands or tens of thousands of people, and aim to confirm and assess the effectiveness of the vaccine and identify any rare side effects which only show up in large groups. The World Health Organization (WHO) lists vaccine candidates at various stages of clinical trials. Here is a slightly more detailed look at those vaccines that have made it to phase 1 and beyond, based on publicly available information.
PHASE 3
- MODERNA (USA) – MRNA VACCINE
This RNA vaccine, which is designed to induce antibodies against a portion of the coronavirus ‘spike’ protein, has been developed by Moderna, in Cambridge, Massachusetts, with funding from the National Institute of Allergy and Infectious Diseases (NIAID) – part of the US National Institutes of Health. It has already been tested in people of various ages and a phase 3 trial, which will enrol 30,000 healthy people from across the United States, started in July.
- SINOVAC (CHINA) – INACTIVATED VACCINE
This vaccine uses an inactivated form of the SARS-CoV-2 virus to try and induce immunity. It protected rhesus macaques from infection when they were tested three weeks after immunisation, and Sinovac recently began phase 3 trials involving 9,000 volunteers in Brazil and Indonesia. The Chinese government has granted the Sinovac vaccine emergency approval for limited use, a report in July said.
- WUHAN INSTITUTE OF BIOLOGICAL PRODUCTS (CHINA) – INACTIVATED VACCINE
The Wuhan Institute is part of the state-owned Chinese company Sinopharm and has been putting this inactivated (killed virus) vaccine through clinical tests. It launched phase 3 trials in the United Arab Emirates in July, and in Peru and Morocco in August.
- BEIJING INSTITUTE OF BIOLOGICAL PRODUCTS (CHINA) – INACTIVATED VACCINE
The Beijing Institute is also part of China’s state-run Sinopharm Group, and is collaborating with the Chinese Center for Disease Control and Prevention on this vaccine. Phase 3 trials in 5,000 people in the United Arab Emirates are running alongside those of the Wuhan Institute version.
- CANSINO BIOLOGICS INC. (CHINA) – VIRAL VECTOR VACCINE
This Ad5-nCoV vaccine candidate was jointly developed with the Academy of Military Medical Sciences in China and uses a harmless non-replicating viral vector (a sort of molecular transporter) to carry fragments of viral protein which may stimulate an immune response into the human body.
The Tianjin-based company used the same platform for its Ebola vaccine. In July, CanSino Biologics reported that phase 2 trials of the new vaccine showed that it produced a strong immune response. On 9 August, the Saudi health ministry announced that CanSino would run a phase 3 trial of the vaccine in Saudi Arabia; later that month the company also started a trial in Pakistan.
- GAMALEYA RESEARCH INSTITUTE (RUSSIA) – VIRAL VECTOR VACCINE
Gamaleya launched a phase 1 clinical trial on this non-replicating viral vector vaccine candidate in mid-June, followed by a phase 3 trial involving more than 2,000 people in Russia, Latin America and the Middle East – smaller than most other phase 3 trials – soon afterwards. In August, Russia had announced that the vaccine would be approved for use, even though these trials have not yet been completed.
PHASE 2/3
- UNIVERSITY OF OXFORD (UK) – VIRAL VECTOR VACCINE
Phase 3 clinical trials of the ChAdOx1 vaccine are ongoing, involving more than 10,000 volunteers from across the UK, including children and older people. It is also being tested in Brazil, the United States and South Africa – the first COVID-19 vaccine trial in Africa. The vaccine was developed by the University of Oxford and AstraZeneca has agreed to manufacture 300 million doses, with support from the Coalition for Epidemic Preparedness Innovations (CEPI). Assuming it is safe and effective, the first doses are expected to be available in late 2020. However, on 9 Sept the decision was made to halt the trial after on volunteer experienced an unexplained illness.
- BIONTECH (GERMANY) – MRNA VACCINE
Working with Pfizer, BioNTech is currently testing its BNT162 vaccine in human volunteers in Germany and the United States. It announced the launch of a phase 2/3 trial involving 30,000 volunteers in the United States, Argentina, Brazil and Germany on 27 July. The company has also entered into a €100 million debt financing agreement with the European Investment Bank to scale-up European production of the vaccine.
dott. Dario Sannino
